Regulations Compliance
Data Security & Compliance
Improve your lab compliancy by using LabCollector to handle 21 CFR 11, Annex 11, GxP and ISO rules
Data Security
User Access Management
You have full control over who can access your data. User and Group policies enable you to assign specific access privileges to users and groups, ensuring that only authorized personnel have access to your data. Our LDAP/AD and SSO features simplify the management of user authentication and authorization.
Data Backup and Recovery
Set up regular backups to ensure that your data is protected in the event of an unforeseen event such as a system failure or data corruption. Our data recovery process is quick and hassle-free, so you can get back to work with minimal disruption.
Audit Trail
An audit trail records all user activity within the system to ensure complete transparency and accountability. This feature allows you to track who accessed your data, when they did it, and what changes were made, providing you with a clear view of your data’s lifecycle.
Data Encryption
We use encryption to protect your data. Our encrypted database and HTTPS options on the server side ensure that your data is securely transmitted and stored.
Two-Factor Authentication and CAPTCHAs
We offer 2FA and CAPTCHA options. Two-factor authentication adds an extra layer of security to your account, ensuring that only authorized personnel can log in. CAPTCHAs, on the other hand, prevent automated attacks and ensure that the person accessing the system is human.
Infra/Code-side
We understand that security is a continuous process, and we regularly perform pentests and security audits to ensure the application and code remain secure.
Data Inventory Compliance
Archive of records
A record can be easily archived with a single click. The record will not be displayed in the search results unless a specific filter is applied. Modification will not be allowed in archived records and can only be duplicated, printed or stored. Records with storage or linked to another element cannot be archived.
Reasons to edit a records
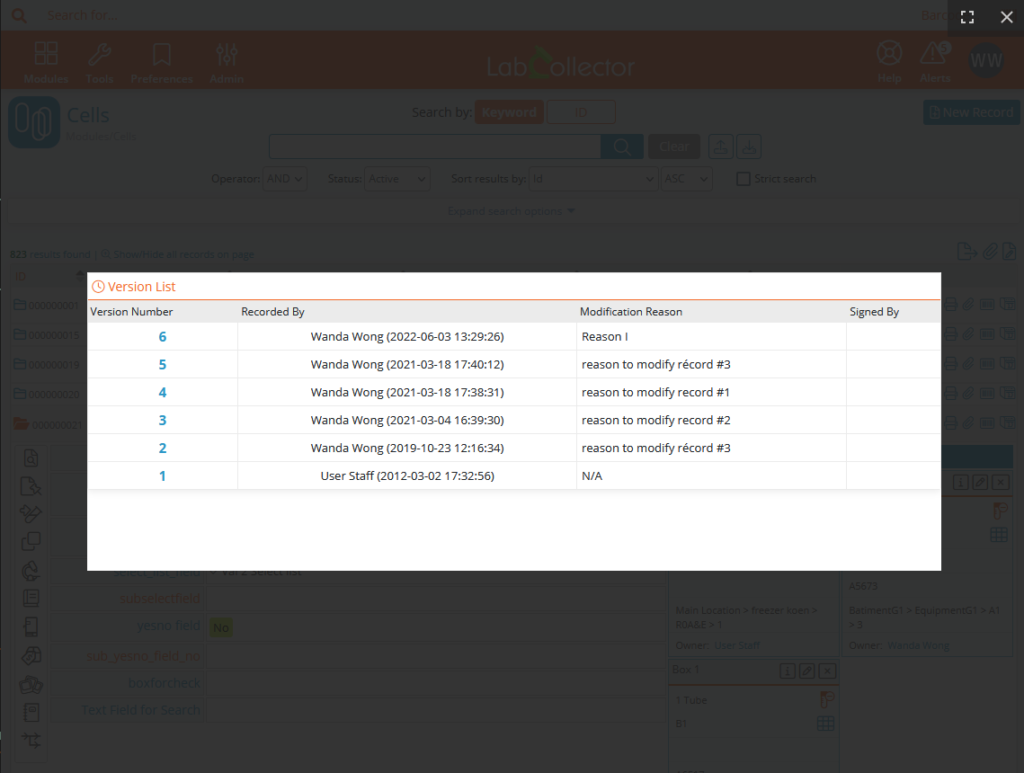
In order to follow and ensure the interest of the modifications made, predefined reasons can be created. These reasons will then be proposed when saving a modification and will appear in the version list and the audit trail. Other reasons can be added manually.
Lock record and read-only function
A record can be locked to avoid further modifications and to ensure its integrity. This lock is visible in the version list.
A read-only record will be displayed in the search results, but no changes will be allowed. This action is irreversible (only the super-administrator can undo it) so the record will always remain in the current version.
Custom fields can be created as read-only fields to avoid any modification of this predefined field.
Only available in the Compliancy Pack – Contact us for more information
Records & custom fields’ versioning
Versioning keeps track of all changes saved to the record. Changes are also tracked in the audit trail. However, versioning provides a simple method to quickly access information on a record-by-record basis.
Only available in the Compliancy Pack – Contact us for more information
Encrypted custom fields
Data in a custom field type text can be encrypted in the database and is only visible in the UI. Search filters and searchable options are not possible in this case.
Only available in the Compliancy Pack – Contact us for more information

Other Compliance Features
Audit Trail Log
LabCollector allows you to have audit trail log for all the changes made in the LabCollector. In order to find the information without difficulties, it is possible to search by keyword, date, day or by module.
E-signature of records and reasons to sign
Electronic signature on blocked records allows to guarantee the integrity of the data. Signatures are shown in the version list and the audit trail. In order to ensure effective data tracking, the user must provide a reason for signing this record. Pre-defined reasons can be created. These reasons will be visible in the versioning and audit trail.
Only available in the Compliancy Pack – Contact us for more information
Login options
Login options will show you different options to login and also allow you to configure the LADP/AD and Single Sign On (SSO) authentication function. Other options are available like Two Factor Authentication or Captcha test. More stringent password rules are possible if you decide to enable this feature.
Compliance with Electronic Lab Notebook
The ELN add-on offers by default many options for tracking changes to ensure data integrity and no loss of information:
- The audit trail log allows you to see all modifications done by the selected user, in a specific date range
- The log activity provides a simplified version of the last 50 entries
- The time function can be a real project/team management tool. You can visualize the time required to perform project tasks. You can collect data (task time and each user’s time per project) by exporting an Excel file.
ELN e-signature
LabCollector’s ELN includes an easy-to-use electronic signature validation system that allows data integrity check (based on CFR 21 Part 11 and patents requirements). Notebook pages can be signed by both the authors and admin users. E-signatures give proof of content validity at signature date.
This option must be purchased
Compliance with Lab Service Manager
Lab Service Manager has its own rules of regulation in addition to the basic rules.
- An audit trail log to follow up user activities in the LSM
- A Compliance function in LSM can be activated with one click and configured according to your needs
NOTE
LabCollector can help with compliance if used correctly.
Compliance requires a thorough reading of the appropriate regulations. It is up to each laboratory to determine which regulations require specific training or practices. The responsibility for some regulations related to the use of software and electronic records rests primarily or entirely with a laboratory and not with the software that is used in the laboratory.
Many aspects of compliance require LabCollector end-users to take on certain responsibilities, such as training staff in certain concepts, which is not possible for the software. LabCollector users should always review the appropriate regulations and guidance documents that apply to a particular situation to ensure compliance.
You might also be interested in our Training Manager add-on, an integrated training and competencies solution to quickly enforce staff skills and meet regulatory requirements. Read more here.


